Have you ever wondered why some foods digest faster than others? The answer lies in the fascinating world of enzyme activity. In this article, we will dive deep into the factors that affect enzymatic efficiency and shed light on how these catalysts work their magic in our bodies. Whether you're a health-conscious individual seeking to optimize your nutrition or simply intrigued by the science of digestion, join us on this journey as we uncover the key elements that influence enzyme activity and their impact on our overall health.
What Is Enzyme Activity?
Enzyme activity refers to the ability of enzymes to catalyze or accelerate chemical reactions in living organisms. Enzymes are biological molecules that act as catalysts, meaning they facilitate the conversion of substrates into products without being consumed in the process. Enzymes achieve this by lowering the activation energy required for a reaction to occur, thereby increasing the rate of the reaction. Enzyme activity is vital for numerous biological processes, including digestion, metabolism, DNA replication, and cellular signaling. Understanding and optimizing enzyme activity is crucial for maintaining overall health and well-being.
What Factors Affect Enzyme Activity?
Temperature
Temperature plays a crucial role in enzyme activity. As the temperature increases, the rate of enzyme-catalyzed reactions generally increases as well. This is because higher temperatures provide more energy for the reactant molecules, allowing them to collide more frequently and with greater force. However, extreme temperatures can denature enzymes, causing them to lose their shape and function. Therefore, maintaining an optimal temperature range is essential for maximizing enzymatic efficiency.
pH Levels
The pH level, or acidity/alkalinity, of the environment also affects enzyme activity. Enzymes have an optimal pH range in which they function most effectively. Deviating from this range can disrupt the enzyme's structure and alter its ability to bind with the substrate. Different enzymes have different pH optima, so understanding the pH requirements of specific enzymes is crucial for maintaining their activity.
Substrate Concentration
The concentration of the substrate, or the molecule that the enzyme acts upon, can significantly impact enzyme activity. Initially, as substrate concentration increases, the rate of the reaction also increases, as more substrate molecules are available for the enzyme to bind with. However, there comes a point where all the enzyme molecules are occupied, and adding more substrate does not increase the reaction rate. This is known as reaching the enzyme's saturation point.
Enzyme Concentration
The concentration of enzymes in a reaction also affects the rate of enzyme activity. Generally, as the enzyme concentration increases, the reaction rate increases as well, assuming that there is an excess of substrate available. More enzymes mean more active sites available for substrate binding, leading to a higher rate of product formation. However, once the substrate becomes limiting, increasing the enzyme concentration will not further increase the reaction rate.
Presence of Inhibitors
Inhibitors are molecules that can bind to enzymes and reduce their activity. They can either bind to a different site on the enzyme and change its shape so that it can not bind to the substrate (competitive inhibitors) or they can compete with the substrate for the active site. The presence of inhibitors can significantly impact enzyme activity, reducing the rate of the reaction or completely stopping it.
Cofactors and Coenzymes
Some enzymes require additional non-protein molecules, known as co-factors or co-enzymes, to function properly. These molecules can be inorganic ions or organic compounds and are essential for the enzyme's catalytic activity. Co-factors and co-enzymes can help the enzyme bind to substrates, take part in chemical reactions, or move functional groups around inside the active site of the enzyme.
Enzyme-Substrate Specificity
Enzymes exhibit specificity towards their substrates, meaning they are designed to bind and act upon specific molecules. The active site of the enzyme and the substrate's complementary shape and chemical properties determine this specificity. The enzyme-substrate specificity is crucial for efficient catalysis and prevents unwanted reactions with other molecules.
Genetic Factors
Genetic factors can also affect enzyme activity. Variations in the genes that code for enzymes can result in different enzyme structures or levels of enzyme expression. These genetic variations can affect the efficiency and functionality of enzymes, leading to individual differences in enzyme activity and metabolism.
Environmental Factors
Apart from temperature and pH, other environmental factors such as pressure, humidity, and the presence of certain chemicals, factors, can also impact enzyme activity. Extreme conditions or the presence of substances that interfere with enzyme function can disrupt the enzyme's structure and affect its activity.
Enzyme Regulation
Enzyme activity can be regulated through various mechanisms, including feedback inhibition, allosteric regulation, and post-translational modifications. These regulatory mechanisms allow cells to control enzyme activity based on the metabolic needs of the organism, ensuring that enzymes are active when required and inactive when not needed.
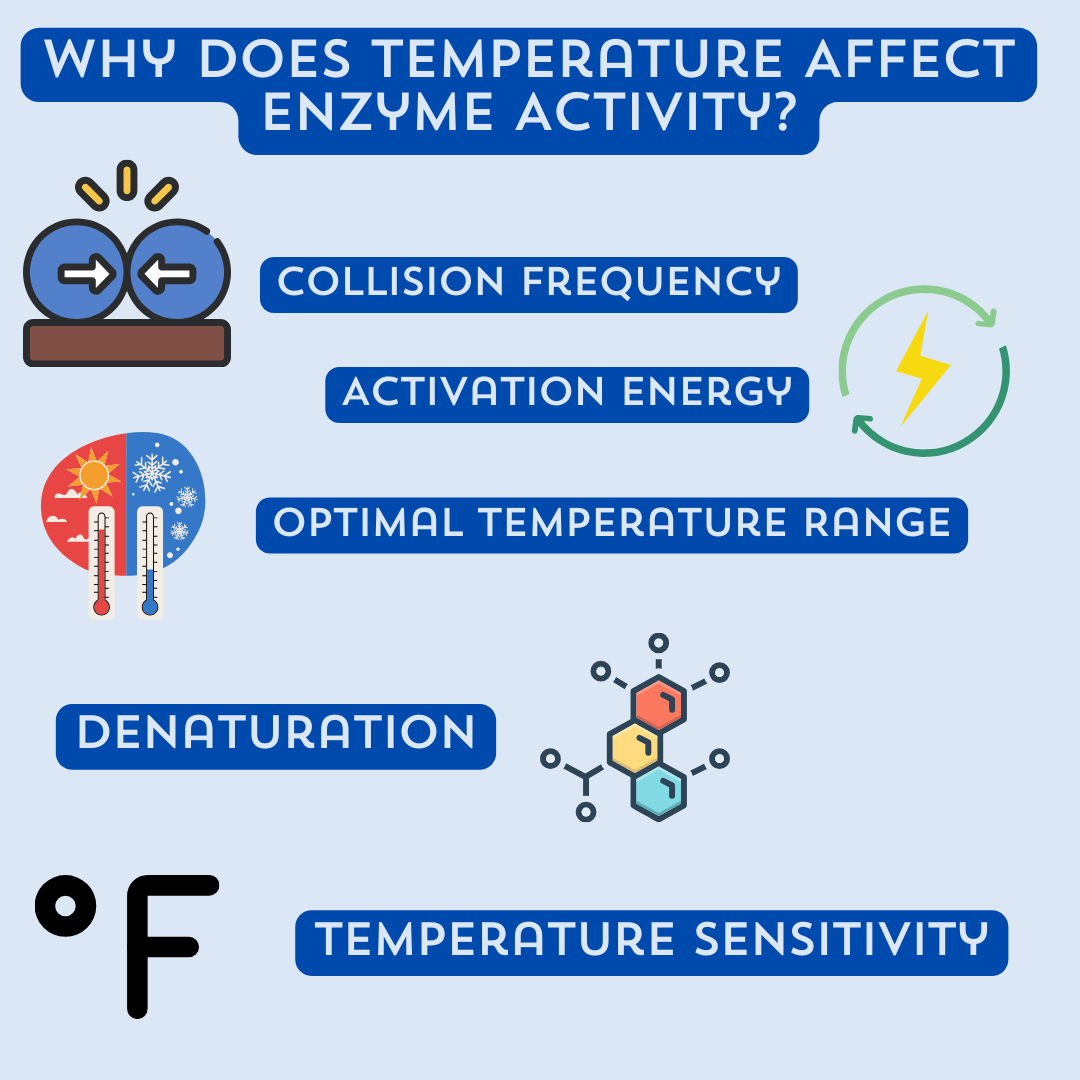
Why Does Temperature Affect Enzyme Activity?
Temperature affects enzyme activity due to the following reasons:
- Collision Frequency: Increasing temperature leads to higher molecular motion and collision frequency among enzyme and substrate molecules. This enhances the chances of successful collisions and formation of enzyme-substrate complexes, thereby increasing the rate of enzyme activity.
- Activation Energy: Enzymes work by lowering the activation energy required for a chemical reaction to occur. Higher temperatures provide more thermal energy, which helps overcome the activation energy barrier, allowing reactions to proceed at a faster rate.
- Optimal Temperature Range: Enzymes have an optimal temperature range at which they exhibit maximum activity. This range varies for different enzymes. Deviating from this range can denature the enzyme, causing it to lose its shape and function, leading to a decrease in enzymatic activity.
- Denaturation: Extreme temperatures can disrupt the weak bonds and interactions that maintain the enzyme's three-dimensional structure. This denaturation results in the loss of enzyme activity as the active site becomes distorted, preventing proper substrate binding and catalysis.
- Temperature Sensitivity: Enzymes have specific temperature sensitivities. Some enzymes are more active at lower temperatures (cold-adapted enzymes), while others function optimally at higher temperatures (thermophilic enzymes). Understanding the temperature sensitivity of enzymes is crucial for optimizing their activity in various biological processes and industrial applications.
How Does pH Affect Enzyme Activity?
pH plays a critical role in enzyme activity as it affects the enzyme's structure and the chemical properties of the substrate. Enzymes have an optimal pH range at which they exhibit maximum activity. Deviating from this range can lead to a decrease in enzyme activity.
The pH affects enzyme activity by influencing the ionization state of amino acid residues within the enzyme's active site. These residues are responsible for substrate binding and catalysis. Changes in pH can disrupt the ionic interactions and hydrogen bonding within the enzyme, altering its three-dimensional structure and, subsequently, its ability to bind with the substrate.
Additionally, pH can directly affect the charge and solubility of the substrate, influencing its interaction with the enzyme. Therefore, maintaining the appropriate pH conditions is crucial for preserving the enzymatic activity and ensuring optimal performance.
How Does Enzyme Activity Influence Substrate Affinity?
Enzyme activity can significantly influence substrate affinity, which refers to the strength of the binding between an enzyme and its substrate. Higher enzyme activity often correlates with a higher substrate affinity. When an enzyme is highly active, it can efficiently bind with the substrate and catalyze the reaction, resulting in a stronger affinity between the enzyme and substrate.
This enhanced affinity allows the enzyme to effectively recognize and bind with the substrate, leading to a faster reaction rate. On the other hand, lower enzyme activity may result in reduced substrate affinity, as the enzyme may have difficulty binding with the substrate or catalyzing the reaction efficiently. Therefore, the level of enzyme activity directly impacts the strength of the enzyme-substrate interaction and ultimately influences the substrate affinity.
How Does Enzyme Activity Interact With Proteins?
Enzyme activity can interact with proteins in various ways, playing a crucial role in numerous biological processes. Enzymes themselves are proteins that act as catalysts, facilitating biochemical reactions in living organisms. They can interact with other proteins through processes such as protein-protein interactions, allosteric regulation, and enzyme-substrate binding. Enzymes can bind to specific proteins to modify their structure or function, regulate their activity, or participate in complex signaling pathways. Additionally, enzymes can also be regulated by proteins, such as inhibitors or activators, which can modulate their activity levels.
Conclusion
In conclusion, understanding the factors that affect enzyme activity is crucial for optimizing enzymatic efficiency and maximizing their role in biological processes. Temperature and pH levels play a significant role in determining enzyme activity, with deviations from optimal ranges leading to decreased efficiency. Substrate concentration and enzyme concentration also impact enzyme activity, with saturation points and limiting factors influencing reaction rates. The presence of inhibitors, co-factors, and co-enzymes further modulate enzyme activity. Genetic factors, environmental conditions, and enzyme regulation mechanisms add additional layers of complexity to enzyme activity. By comprehending these factors and their interplay, we can harness the power of enzymes to enhance our understanding of biochemical processes and improve various aspects of health and industry. With this knowledge, we can make informed choices to optimize our wellness and maintain a healthy lifestyle.
Final Thoughts
Unlock your body's full potential with World Nutrition's Vitalzym Extra Strength, the leading vegetarian systemic enzyme supplement. Designed for maximum effectiveness, Vitalzym supports enzyme restoration, immune health, post-exercise recovery, and healthy circulation. Featuring a potent blend of serrapeptase, bromelain, papain, and more, our formula promotes overall vitality and well-being. Don't let aging or lifestyle challenges slow you down. Explore World Nutrition products and take the first step towards a vibrant, healthier you.*
Sources
- https://www.pminstrumentation.co.za/the-impact-of-humidity-on-food/
- https://www.bbc.co.uk/bitesize/guides/z9jrng8/revision/3
- https://www.ucl.ac.uk/~ucbcdab/enzass/substrate.htm
- https://www2.nau.edu/lrm22/lessons/enzymes/enzymes.html
These statements have not been evaluated by the food and drug administration (FDA). These products are not intended to diagnose, treat, cure, or prevent any disease.